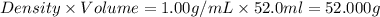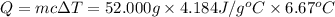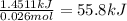Chemistry, 05.07.2019 19:10 angelolucero146
When 26.0 ml of 0.500 m h2so4 is added to 26.0 ml of 1.00 m koh in a coffee-cup calorimeter at 23.50°c, the temperature rises to 30.17°c. calculate δh of this reaction. (assume that the total volume is the sum of the individual volumes and that the density and specific heat capacity of the solution are the same as for pure water.) (d for water = 1.00 g/ml; c for water = 4.184 j/g·°c.)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 13:20
An aqueous solution of calcium hydroxide is standardized by titration with a 0.120 m solution of hydrobromic acid. if 16.5 ml of base are required to neutralize 27.5 ml of the acid, what is the molarity of the calcium hydroxide solution?
Answers: 3

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 01:30
Asap! how do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 1

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2
You know the right answer?
When 26.0 ml of 0.500 m h2so4 is added to 26.0 ml of 1.00 m koh in a coffee-cup calorimeter at 23.50...
Questions



English, 10.11.2020 03:00

English, 10.11.2020 03:00

Chemistry, 10.11.2020 03:00






Mathematics, 10.11.2020 03:00


Health, 10.11.2020 03:00

Mathematics, 10.11.2020 03:00






Mathematics, 10.11.2020 03:00

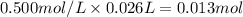
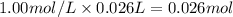
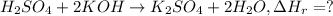
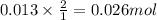 of potassium hydroxide.
of potassium hydroxide.