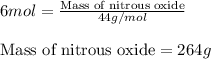Write the balanced chemical equation for the complete, stoichiometric combustion of ethylene in (a) nitrous oxide and (b) air. compare the required number of moles and the oxidizer mass using each of the two oxidizers for the complete, stoichiometric combustion of ethylene.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2


Chemistry, 23.06.2019 03:30
Name 3 types of energy you see being used as you look around a classroom
Answers: 1

You know the right answer?
Write the balanced chemical equation for the complete, stoichiometric combustion of ethylene in (a)...
Questions



Computers and Technology, 27.10.2020 17:50

Mathematics, 27.10.2020 17:50




Computers and Technology, 27.10.2020 17:50










SAT, 27.10.2020 17:50

History, 27.10.2020 17:50

 ....(1)
....(1)