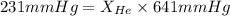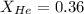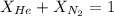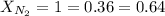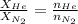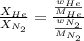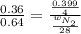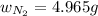Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 23.06.2019 00:30
You are attempting to recrystallize a crude product mixture. you add the appropriate amount of hot solvent and are allowing the solution to slowly cool to room temperature. however, at room temperature no crystals have appeared, which of the following methods should be used to induce crystallization? choose all correct answers. a) place the flask in an ice bath. b) swirl the contents of the flask. c) add a small seed crystal of the desired product. d) scratch the inside of the glassware using a stir rod. it can be multiple choices
Answers: 3

Chemistry, 23.06.2019 04:00
Achemical reaction is done in the setup shown , resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 2
You know the right answer?
Amixture of helium and nitrogen gases at a total pressure of 641 mm mm hg. if the gas mixture contai...
Questions



Mathematics, 15.06.2021 19:50




Mathematics, 15.06.2021 19:50

Arts, 15.06.2021 19:50

Mathematics, 15.06.2021 19:50



World Languages, 15.06.2021 19:50


Chemistry, 15.06.2021 19:50

Physics, 15.06.2021 19:50

Mathematics, 15.06.2021 19:50



Biology, 15.06.2021 19:50

Social Studies, 15.06.2021 19:50

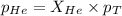
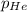 = partial pressure of gas = 231 mmHg
= partial pressure of gas = 231 mmHg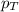 = total pressure of gas = 641 mmHg
= total pressure of gas = 641 mmHg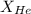 = mole fraction of helium gas = ?
= mole fraction of helium gas = ?