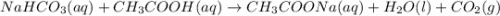Chemistry, 05.07.2019 20:20 jayjinks976
A3.4 g sample of sodium hydrogen carbonate is added to a solution of acetic acid weighing 10.9 g. the two substances react, releasing carbon dioxide gas to the atmosphere. after the reaction, the contents of the reaction vessel weigh 11.6 g. what is the mass of carbon dioxide released during the reaction?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1

Chemistry, 22.06.2019 23:30
The sum of the oxidation numbers in a neutral compound is always
Answers: 2
You know the right answer?
A3.4 g sample of sodium hydrogen carbonate is added to a solution of acetic acid weighing 10.9 g. th...
Questions

Chemistry, 22.12.2020 14:00

Chemistry, 22.12.2020 14:00

Social Studies, 22.12.2020 14:00

Mathematics, 22.12.2020 14:00

English, 22.12.2020 14:00

Social Studies, 22.12.2020 14:00

Physics, 22.12.2020 14:00

Mathematics, 22.12.2020 14:00

Chemistry, 22.12.2020 14:00

Chemistry, 22.12.2020 14:00


Biology, 22.12.2020 14:00

English, 22.12.2020 14:00

World Languages, 22.12.2020 14:00



Mathematics, 22.12.2020 14:00

Physics, 22.12.2020 14:00

Chemistry, 22.12.2020 14:00

Chemistry, 22.12.2020 14:00