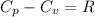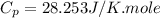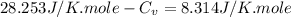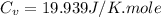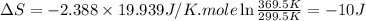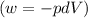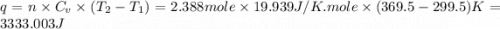Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

Chemistry, 23.06.2019 14:30
An atom of element x has one more shell of electrons than an atom of beryllium, but it has one less valance electron than beryllium. which element is x
Answers: 1
You know the right answer?
Ideal gas (n 2.388 moles) is heated at constant volume from t1 299.5 k to final temperature t2 369.5...
Questions

Mathematics, 15.11.2020 02:20

Mathematics, 15.11.2020 02:20


Mathematics, 15.11.2020 02:20

History, 15.11.2020 02:20

Social Studies, 15.11.2020 02:20

Mathematics, 15.11.2020 02:20

Mathematics, 15.11.2020 02:20

Mathematics, 15.11.2020 02:20


Mathematics, 15.11.2020 02:20






Mathematics, 15.11.2020 02:20



Mathematics, 15.11.2020 02:20

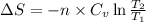
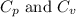 for an ideal gas are :
for an ideal gas are :