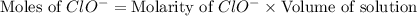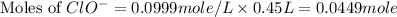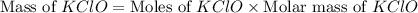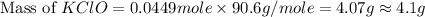Chemistry, 05.07.2019 20:20 TheJanko4526
What mass of potassium hypochlorite (fw-90.6 g/mol) must be added to 4.50 x 10 ml of water to give a solution with ph 10.20? [ka(hcio) 4.0 x 10-8] 0.032g ? 2.4 g 04.1 g 9.1 g 20. g

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3
You know the right answer?
What mass of potassium hypochlorite (fw-90.6 g/mol) must be added to 4.50 x 10 ml of water to give a...
Questions

Mathematics, 01.09.2020 04:01

Mathematics, 01.09.2020 04:01

Mathematics, 01.09.2020 04:01



Mathematics, 01.09.2020 04:01

Mathematics, 01.09.2020 04:01


Mathematics, 01.09.2020 04:01


Mathematics, 01.09.2020 04:01


Health, 01.09.2020 04:01

Mathematics, 01.09.2020 04:01

Mathematics, 01.09.2020 04:01





Engineering, 01.09.2020 04:01

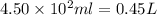
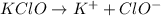
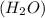 to give,
to give,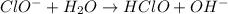
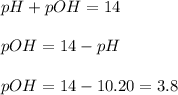
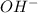 concentration.
concentration.![pOH=-\log [OH^-]](/tpl/images/0055/2981/1fac1.png)
![3.8=-\log [OH^-]](/tpl/images/0055/2981/a714c.png)
![[OH^-]=1.58\times 10^{-4}M](/tpl/images/0055/2981/110da.png)
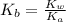
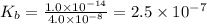
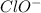 .
.![K_b=\frac{[OH^-][HClO]}{[ClO^-]}](/tpl/images/0055/2981/9b5c8.png)
![[OH^-]=[HClO]=1.58\times 10^{-4}M](/tpl/images/0055/2981/ec220.png)
![2.5\times 10^{-7}=\frac{(1.58\times 10^{-4})^2}{[ClO^-]}](/tpl/images/0055/2981/2ef50.png)
![[ClO^-]=0.0999M](/tpl/images/0055/2981/776df.png)