
The equilibrium constant, kc, for the following reaction is 9.52×10-2 at 350 k. ch4 (g) + ccl4 (g) 2 ch2cl2 (g) calculate the equilibrium concentrations of reactants and product when 0.377 moles of ch4 and 0.377 moles of ccl4 are introduced into a 1.00 l vessel at 350 k.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2


Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2
You know the right answer?
The equilibrium constant, kc, for the following reaction is 9.52×10-2 at 350 k. ch4 (g) + ccl4 (g) 2...
Questions

History, 07.04.2021 07:30

Biology, 07.04.2021 07:30



Mathematics, 07.04.2021 07:30


Chemistry, 07.04.2021 07:30



Chemistry, 07.04.2021 07:30

Mathematics, 07.04.2021 07:30


English, 07.04.2021 07:30

Mathematics, 07.04.2021 07:30

Chemistry, 07.04.2021 07:30


History, 07.04.2021 07:30



Mathematics, 07.04.2021 07:30

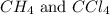 is 0.377 M and equilibrium concentration of
is 0.377 M and equilibrium concentration of 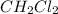 is 0.116 M
is 0.116 M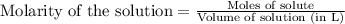
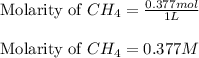

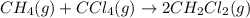
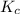 for the given equation follows:
for the given equation follows:![K_c=\frac{[CH_2Cl_2]^2}{[CH_4][CCl_4]}](/tpl/images/0055/3628/bf52a.png)
![K_c=9.52\times 10^{-2}\\[CH_4]=0.377M\\[CCl_4]=0.377M](/tpl/images/0055/3628/8ee11.png)
![9.52\times 10^{-2}=\frac{[CH_2Cl_2]^2}{(0.377)\times (0.377)}](/tpl/images/0055/3628/5aafe.png)
![[CH_2Cl_2]=0.116M](/tpl/images/0055/3628/7ed7d.png)


