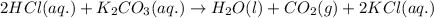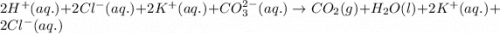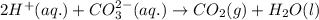Chemistry, 05.07.2019 22:10 chloeholt123
Enter the balanced complete ionic equation for hcl(aq)+k2co3(aq)→h2o(l)+co2(g)+kcl (aq). express your answer as a chemical equation. identify all of the phases in your answer.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1


Chemistry, 23.06.2019 00:00
How many atoms or molecules are there in a mole of a substance?
Answers: 1
You know the right answer?
Enter the balanced complete ionic equation for hcl(aq)+k2co3(aq)→h2o(l)+co2(g)+kcl (aq). express you...
Questions

English, 01.04.2020 22:01


Geography, 01.04.2020 22:01

Social Studies, 01.04.2020 22:01

Mathematics, 01.04.2020 22:01

History, 01.04.2020 22:01

Mathematics, 01.04.2020 22:01


Mathematics, 01.04.2020 22:01





Computers and Technology, 01.04.2020 22:02



Mathematics, 01.04.2020 22:02

English, 01.04.2020 22:02