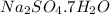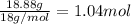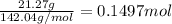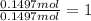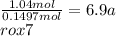Chemistry, 05.07.2019 22:10 thesleepycat
A40.15 gram sample of a hydrate of na2so4 was heated thoroughly in a porcelain crucible, until its weight remained constant. after heating, 21.27 grams of the anhydrous compound remained. what is the formula of the hydrate?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 1

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1
You know the right answer?
A40.15 gram sample of a hydrate of na2so4 was heated thoroughly in a porcelain crucible, until its w...
Questions

Biology, 29.03.2020 19:58

Chemistry, 29.03.2020 19:58


Mathematics, 29.03.2020 19:58






Social Studies, 29.03.2020 19:59

Physics, 29.03.2020 19:59


Mathematics, 29.03.2020 19:59

Mathematics, 29.03.2020 19:59

Computers and Technology, 29.03.2020 20:00


Spanish, 29.03.2020 20:00