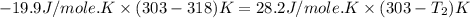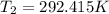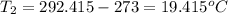Chemistry, 05.07.2019 22:30 itaheart101
Two systems with heat capacities 19.9 j mol-1 k-1 and 28.2 ] mol 1 k-1 respectively interact thermally and come to an equilibrium temperature of 300c. if the initial temperature of system 1 was 450c, what was the initial temperature of system 2 in °c? you may assume that the total energy of the combined systems remains constant

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:10
Which equation represents a fission reaction? o "9n+h—150 o 235u + n—190cs + rb+25 o be + he—1c + in o 28 np —> 2390 pute
Answers: 1

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3
You know the right answer?
Two systems with heat capacities 19.9 j mol-1 k-1 and 28.2 ] mol 1 k-1 respectively interact thermal...
Questions

Mathematics, 24.07.2019 22:00



Mathematics, 24.07.2019 22:00

Mathematics, 24.07.2019 22:00



English, 24.07.2019 22:00


Social Studies, 24.07.2019 22:00

Social Studies, 24.07.2019 22:00

History, 24.07.2019 22:00


Social Studies, 24.07.2019 22:00




Mathematics, 24.07.2019 22:00


History, 24.07.2019 22:00

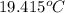
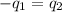
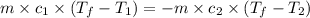
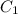 = heat capacity of system 1 = 19.9 J/mole.K
= heat capacity of system 1 = 19.9 J/mole.K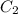 = heat capacity of system 2 = 28.2 J/mole.K
= heat capacity of system 2 = 28.2 J/mole.K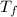 = final temperature of system =
= final temperature of system = 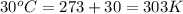
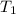 = initial temperature of system 1 =
= initial temperature of system 1 = 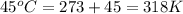
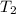 = initial temperature of system 2 = ?
= initial temperature of system 2 = ?