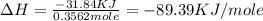Find the enthalpy of neutralization of hcl and naoh. 137 cm3 of 2.6 mol dm-3 hydrochloric acid was neutralized by 137 cm3 of 2.6 mol dm-3 naoh. the temperature rose from 298 k to 325.8 k. the specific heat capacity is the same as water, 4.18 j/k g.
a. 44.69 kj/mol
b. 6123.06 kj/mol
c. 597.46 kj/mol
d. 89.39 kj/mol

Answers: 2
Another question on Chemistry

Chemistry, 23.06.2019 00:00
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2

Chemistry, 23.06.2019 14:30
Will give imagine you are given a mystery element. it is, however, a discovered and known element. you may perform a maximum of two observations or tests to determine its identity. time and money is critical, so you need to prioritize your tests. if you can get by with a single test, you get 100 super-geek points from your research lab team. pick your two tests, number them as #1 and #2, and justify why you think these two will certainly be enough (and why the first might well be enough all by itself.) the available tests are classification into metal, non-metal, or metalloid, count of valence electrons, count of electron shells, atomic radius (error range: +/- 1 pm), electronegativity (error range: +/- 0.1), first ionization energy (error range: +/- 10 kj/mole), melting point (error range: +/- 10 c), and boiling point (error range: +/- 20 c).
Answers: 2

Chemistry, 24.06.2019 03:00
Which equation represents the total ionic equation for the reaction of hno3 and naoh? • h + oh -> h2o hno3 + naoh — nano3 + h20 h* + no3 + na+ + oh — na + no3 + h20 h* + no3 + oh + h20
Answers: 1

Chemistry, 24.06.2019 04:20
A2.50 g sample of solid sodium hydroxide is added to 55.0 ml of 25 °c water in a foam cup (insulated from the environment) and stirred until it dissolves. what is the final temperature of the solution? δhsoln =-44.51 kj/mol.
Answers: 3
You know the right answer?
Find the enthalpy of neutralization of hcl and naoh. 137 cm3 of 2.6 mol dm-3 hydrochloric acid was n...
Questions

Mathematics, 16.11.2020 02:30

Mathematics, 16.11.2020 02:30


Mathematics, 16.11.2020 02:30


Physics, 16.11.2020 02:30



Mathematics, 16.11.2020 02:30










English, 16.11.2020 02:30

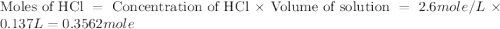
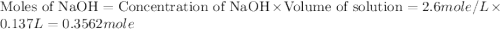

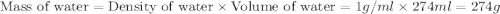
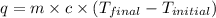
 = specific heat of water =
= specific heat of water = 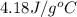
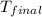 = final temperature of water = 325.8 K
= final temperature of water = 325.8 K = initial temperature of metal = 298 K
= initial temperature of metal = 298 K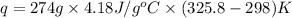
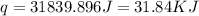
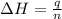
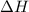 = enthalpy of neutralization = ?
= enthalpy of neutralization = ?