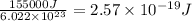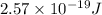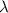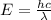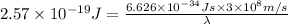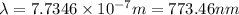Chemistry, 06.07.2019 00:30 Person51761
It takes 155. kj/mol to break a fluorine-fluorine single bond. calculate the maximum wavelength of light for which a flouine-flouring single bond could be broken by absorbing a single photon

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2



You know the right answer?
It takes 155. kj/mol to break a fluorine-fluorine single bond. calculate the maximum wavelength of l...
Questions






Chemistry, 21.07.2019 14:30

Mathematics, 21.07.2019 14:30




English, 21.07.2019 14:30

Social Studies, 21.07.2019 14:30




Social Studies, 21.07.2019 14:30

Mathematics, 21.07.2019 14:30


History, 21.07.2019 14:30

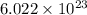 molecules = 155000 J
molecules = 155000 J