
Chemistry, 06.07.2019 03:20 yazmineespinozarive
Determine the amount of heat (in kj) given off when 1.26 × 104 g of ammonia are produced according to the equation n2(g) + 3h2(g) ⟶ 2nh3(g) δh°rxn = −92.6 kj/mol assume that the reaction takes place under standardstate conditions at 25°c.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 23.06.2019 00:00
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2
You know the right answer?
Determine the amount of heat (in kj) given off when 1.26 × 104 g of ammonia are produced according t...
Questions

Mathematics, 22.10.2020 02:01


Health, 22.10.2020 02:01



Social Studies, 22.10.2020 02:01




Mathematics, 22.10.2020 02:01






Geography, 22.10.2020 02:01

Engineering, 22.10.2020 02:01

Mathematics, 22.10.2020 02:01


Mathematics, 22.10.2020 02:01

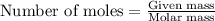
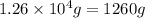
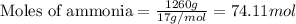
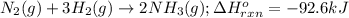
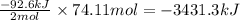 of energy.
of energy.

