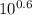Chemistry, 30.01.2020 02:03 lewisf2578
Calculation of original ph from final ph after titration a biochemist has 100 ml of a 0.10 m solution of a weak acid with a pka of 6.3. she adds 6.0 ml of 1.0 m hcl, which changes the ph to 5.7. what was the ph of the original solution?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:10
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3


Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1
You know the right answer?
Calculation of original ph from final ph after titration a biochemist has 100 ml of a 0.10 m solutio...
Questions

Mathematics, 18.08.2019 16:00




Mathematics, 18.08.2019 16:00

Mathematics, 18.08.2019 16:00



Physics, 18.08.2019 16:00


English, 18.08.2019 16:00

Mathematics, 18.08.2019 16:00

Physics, 18.08.2019 16:00




Mathematics, 18.08.2019 16:00

Mathematics, 18.08.2019 16:00

Biology, 18.08.2019 16:00

Mathematics, 18.08.2019 16:00