
Chemistry, 06.07.2019 05:20 baeethtsadia
The wavelength of the red-pink line emitted by a laboratory sample of excited hydrogen is 656 nm. taking a spectrum of a glowing nebula, you find that the same red-pink line of hydrogen appears at 662 nm. you conclude that the nebula
a. is 1% hotter than hydrogen in the laboratory sample.
b. is moving towards us at about 1% the speed of light.
c. is 1% cooler than hydrogen in the laboratory sample.
d. is moving away from us at about 1% the speed of light

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:30
**40** points asapessay questions (10 points possible) clear image of next, create your own scenario. it can be one of your own real experiences or one you make up. use imagery in your writing to give your instructor a the setting and an action taking pace in your writing explain the structure and functions of the skin at work in your scenario. !
Answers: 3


Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3
You know the right answer?
The wavelength of the red-pink line emitted by a laboratory sample of excited hydrogen is 656 nm. ta...
Questions

Chemistry, 20.02.2020 02:47





Mathematics, 20.02.2020 02:48

Mathematics, 20.02.2020 02:48


Biology, 20.02.2020 02:48


Business, 20.02.2020 02:48





History, 20.02.2020 02:48



Mathematics, 20.02.2020 02:48

History, 20.02.2020 02:48

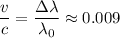 .
.

