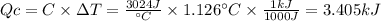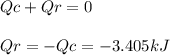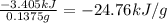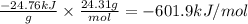Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

Chemistry, 22.06.2019 21:00
Acandle’s wick is the fabric string that holds the flame, and it burns down at a constant slow pace when the candle is lit. the wick is usually surrounded by wax. which is the most important property of covalent compounds that makes them useful for making candle wax? a low boiling point a low melting point a high boiling point a high melting point
Answers: 1
You know the right answer?
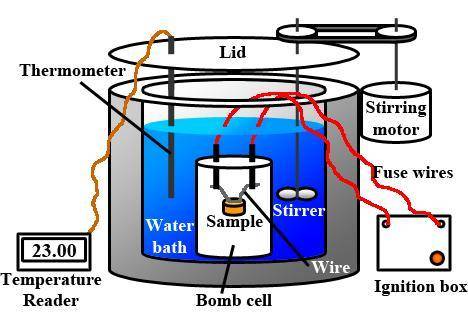
A0.1375-g sample of solid magnesium is burned in a constant-volume bomb calorimeter that has a heat...
Questions

History, 28.10.2020 17:50

Mathematics, 28.10.2020 17:50


Biology, 28.10.2020 17:50


English, 28.10.2020 17:50

Mathematics, 28.10.2020 17:50

History, 28.10.2020 17:50






Geography, 28.10.2020 17:50



Mathematics, 28.10.2020 17:50



Mathematics, 28.10.2020 17:50