
Which of the following would increase the rate of a chemical reaction between hydrochloric acid (hcl) and solid zinc metal (zn)?
a. decreasing the concentration of hcl
b. pulverizing the zinc metal into a fine powder
c. performing the reaction at a lower temperature
d. decreasing the amount of zn

Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3
You know the right answer?
Which of the following would increase the rate of a chemical reaction between hydrochloric acid (hcl...
Questions




Geography, 20.10.2019 04:10



Advanced Placement (AP), 20.10.2019 04:10


Mathematics, 20.10.2019 04:10

Mathematics, 20.10.2019 04:10


History, 20.10.2019 04:10




Physics, 20.10.2019 04:10

Mathematics, 20.10.2019 04:10

Mathematics, 20.10.2019 04:10


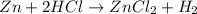
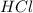 and
and 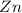 : Thus rate of the reaction would decrease on decreasing the concentration of hydrochloric acid and zinc.
: Thus rate of the reaction would decrease on decreasing the concentration of hydrochloric acid and zinc.

