
Chemistry, 05.02.2020 12:00 hillisaiah734
The reform reaction between steam and gaseous methane (ch4) produces "synthesis gas," a mixture of carbon monoxide gas and dihydrogen gas. synthesis gas is one of the most widely used industrial chemicals, and is the major industrial source of hydrogen.
suppose a chemical engineer studying a new catalyst for the reform reaction finds that 924. liters per second of methane are consumed when the reaction is run at 261.°c and 0.96atm. calculate the rate at which dihydrogen is being produced. give your answer in kilograms per second. round your answer to 2 significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2


Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1
You know the right answer?
The reform reaction between steam and gaseous methane (ch4) produces "synthesis gas," a mixture of c...
Questions


Mathematics, 17.02.2021 17:50

Mathematics, 17.02.2021 17:50

Mathematics, 17.02.2021 17:50



Mathematics, 17.02.2021 17:50


Mathematics, 17.02.2021 17:50





Social Studies, 17.02.2021 17:50

English, 17.02.2021 17:50





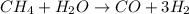 Haber reaction
Haber reaction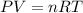
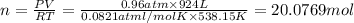
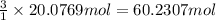 of dihydrogen
of dihydrogen


