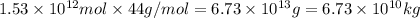Calcium oxide or quicklime (cao) is used in steelmaking, cement manufacture, and pollution control. it is prepared by the thermal decomposition of calcium carbonate: caco3(s) → cao(s) co2(g) calculate the yearly release of co2 (in kg) to the atmosphere if the annual production of cao in the united states is 8.6 × 1010 kg.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:30
For each of the following mixtures decide if filtering would be suitable to separate the substances. explain your answers. oil in water sugar in water sand in water chalk in water tea leaves in a cup of tea
Answers: 2

Chemistry, 21.06.2019 20:40
Which of the following pressures is equal to 760 mm hg? 2.0 atm 101.3 pa 101,300 kpa 101,300 pa
Answers: 2

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1
You know the right answer?
Calcium oxide or quicklime (cao) is used in steelmaking, cement manufacture, and pollution control....
Questions









Mathematics, 22.06.2019 01:20




Mathematics, 22.06.2019 01:20


Mathematics, 22.06.2019 01:20

Mathematics, 22.06.2019 01:20


Mathematics, 22.06.2019 01:20


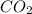 into the atmosphere is
into the atmosphere is 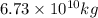 .
.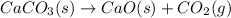
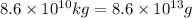
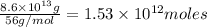
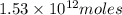 of CaO moles of carbon-dioxide moles produced will be:
of CaO moles of carbon-dioxide moles produced will be: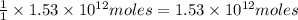 of carbon-dioxide
of carbon-dioxide moles of carbon-dioxide:
moles of carbon-dioxide: