
Chemistry, 08.07.2019 18:10 Shadow0202
The combination of coke and steam produces a mixture called coal gas, which can be used as a fuel or as a starting material for other reactions. if we assume coke can be represented by graphite, the equation for the production of coal gas is
2c(s)+2h2o(> ch4(g)+co2(g)
determine the standard enthalpy change for this reactionf rom the following standard enthalpies of reactions:
c(s)+h2o(> co(g)+h2(g) delta h=131.3 kj
co(g)+h2o(> co2(g)+h2(g) delta h=-41.2 kj
ch4(g)+h2o(> 3h2(g)+co(g) delta h=206.1 kj

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:50
Suppose you got a low yield of benzoin from your benzoin condensation reaction and thus only have 0.300 g of benzoin to use as the starting material for this reaction. how much concentrated nitric acid should you add? (concentrated nitric acid is 15.8 m). write your answer in the form x.xx ml
Answers: 1

Chemistry, 21.06.2019 21:00
What pressure will be exerted by 0.675 moles of a gas at 25*c if it is in a 0.750-l container?
Answers: 1

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1
You know the right answer?
The combination of coke and steam produces a mixture called coal gas, which can be used as a fuel or...
Questions

Mathematics, 16.04.2020 16:48





Biology, 16.04.2020 16:48


Health, 16.04.2020 16:48







English, 16.04.2020 16:49



Social Studies, 16.04.2020 16:49

Engineering, 16.04.2020 16:49

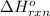 for the reaction is 15.3 kJ.
for the reaction is 15.3 kJ.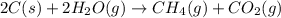
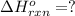

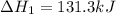 ( × 2)
( × 2)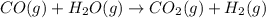


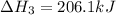
![\Delta H^o_{rxn}=[2\times \Delta H_1]+[1\times \Delta H_2]+[1\times (-\Delta H_3)]](/tpl/images/0066/4813/d71c9.png)
![\Delta H^o_{rxn}=[(2\times (131.3))+(1\times (-41.2))+(1\times (-206.1))]=15.3kJ](/tpl/images/0066/4813/c955f.png)


