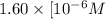Chemistry, 08.07.2019 18:10 PersonPerson13260
Calculate the concentration of h3o⁺ in a solution that contains 6.25 × 10-9 m oh⁻ at 25°c. identify the solution as acidic, basic, or neutral. a) 6.38 × 10-9 m, basic b) 1.60 × 10-6 m, acidic c) 7.94 × 10-11 m, acidic d) 7.38 × 10-3 m, basic e) 4.92× 10-5 m, acidic

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 23.06.2019 02:00
Alice did an experiment to find the relationship between the angle at which a ray of light strikes a mirror and the angle at which the mirror reflects the light. she placed a ray box in front of a mirror. she changed the angle at which the light from the ray box struck the mirror and noted the corresponding angle at which the mirror reflected the light. which of the following is the dependent variable in this experiment? the mirror used to reflect the light the ray box used as the source of light angle at which the light from the ray box strikes the mirror angle at which the mirror reflects the light from the ray box
Answers: 2
You know the right answer?
Calculate the concentration of h3o⁺ in a solution that contains 6.25 × 10-9 m oh⁻ at 25°c. identify...
Questions


History, 05.11.2019 23:31



History, 05.11.2019 23:31




Social Studies, 05.11.2019 23:31

Social Studies, 05.11.2019 23:31

Mathematics, 05.11.2019 23:31




English, 05.11.2019 23:31




English, 05.11.2019 23:31

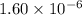 M, acidic
M, acidic![pH=-\log [H_3O^+]](/tpl/images/0066/4841/841e8.png)
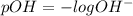
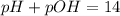
![[OH^-]=6.25\times 10^{-9}M](/tpl/images/0066/4841/a8cb2.png)
![pOH=-log[6.25\times 10^{-9}M]](/tpl/images/0066/4841/b2499.png)
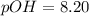
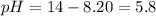
![5.8=-log[H_3O^+]](/tpl/images/0066/4841/59813.png)
![[H_3O^+]=1.60\times [10^{-6}M](/tpl/images/0066/4841/f3b28.png)
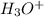 is
is