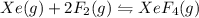Chemistry, 08.07.2019 18:10 alemorachis49
1) consider the following reaction at equilibrium. what effect will reducing the pressure of the reaction mixture have on the system? xe(g) + 2 f2(g) ? xef4(g) a)the equilibrium constant will decrease. b)no effect will be observed. c)the reaction will shift to the right in the direction of products. d) the equilibrium constant will increase e) the reaction will shift to the left in the direction of reactants.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3
You know the right answer?
1) consider the following reaction at equilibrium. what effect will reducing the pressure of the rea...
Questions

Mathematics, 11.07.2019 21:20

Mathematics, 11.07.2019 21:20

Mathematics, 11.07.2019 21:20


Biology, 11.07.2019 21:20



English, 11.07.2019 21:20



English, 11.07.2019 21:20

English, 11.07.2019 21:20





Geography, 11.07.2019 21:20

Social Studies, 11.07.2019 21:20


Mathematics, 11.07.2019 21:20