
Chemistry, 08.07.2019 22:30 kcarstensen59070
The density of water at 4ºc is 1.00 x 103 kg/m3. what is water's density at 82ºc? assume that the water's coefficient of volume expansion is constant. enter your answer in kg/m3, without units, to the nearest whole number.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3

Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
The density of water at 4ºc is 1.00 x 103 kg/m3. what is water's density at 82ºc? assume that the w...
Questions

Mathematics, 11.05.2021 23:20


Arts, 11.05.2021 23:20


Chemistry, 11.05.2021 23:20

Mathematics, 11.05.2021 23:20

Mathematics, 11.05.2021 23:20

Mathematics, 11.05.2021 23:20


Spanish, 11.05.2021 23:20

Mathematics, 11.05.2021 23:20


Spanish, 11.05.2021 23:20



Mathematics, 11.05.2021 23:20




Business, 11.05.2021 23:20

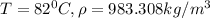
![\rho_1 = \rho_0 [ 1- \beta \Delta T ]](/tpl/images/0067/1477/b4368.png)
 is the density at temperature
is the density at temperature 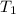
 is the density at temperature
is the density at temperature 
 is the coefficient of volume expansion
is the coefficient of volume expansion  is the change in temperature which is:
is the change in temperature which is: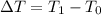

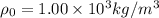


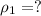


![\rho_1 = 1.00\times 10^3 kg/m^3[1 - 0.000214 ^0C^{-1} \times 78^0 C ]](/tpl/images/0067/1477/6cff4.png)



