
Be sure to answer all parts. nitric oxide (no) reacts with oxygen gas to form nitrogen dioxide (no2), a dark brown gas: 2no(g) + o2(g) → 2no2(g)
in one experiment, 0.857 mol of no is mixed with 0.498 mol of o2.
determine which of the two reactants is the limiting reactant. calculate also the number of moles of no2 produced. limiting reactant: moles of no2 produced: moles

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 22.06.2019 23:00
What is formed when amino acids form long chains or polymerize
Answers: 1

Chemistry, 23.06.2019 07:00
What is the difference between covalent bonds and ionic bonds? covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the electrical attraction between charged atoms. covalent bonds involve the transfer of electrons between charged atoms; ionic bonds involve the sharing of electrons between atoms. covalent bonds involve the sharing of pairs of electrons between atoms; ionic bonds involve the sharing of single electrons between atoms. covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the sharing of protons between charged atoms.
Answers: 1
You know the right answer?
Be sure to answer all parts. nitric oxide (no) reacts with oxygen gas to form nitrogen dioxide (no2)...
Questions

Biology, 04.08.2019 12:00


Chemistry, 04.08.2019 12:00

History, 04.08.2019 12:00



Mathematics, 04.08.2019 12:00






Social Studies, 04.08.2019 12:00


Physics, 04.08.2019 12:00




Biology, 04.08.2019 12:00

Mathematics, 04.08.2019 12:00

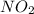 will be produced.
will be produced.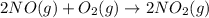
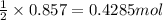 of
of 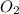
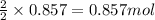 of
of 

