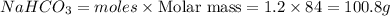Chemistry, 08.07.2019 23:40 skinniestoflegends
Be sure to answer all parts. when baking soda (sodium bicarbonate or sodium hydrogen carbonate, nahco3) is heated, it releases carbon dioxide gas, which is responsible for the rising of cookies, donuts, and bread. write a balanced equation for the decomposition of the compound (one of the three products is na2co3). do not include states of matter in your balanced equation. calculate the mass of nahco3 required to produce 27.0 g of co2. g nahco3

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1


Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3
You know the right answer?
Be sure to answer all parts. when baking soda (sodium bicarbonate or sodium hydrogen carbonate, nahc...
Questions




Mathematics, 06.01.2021 21:30

Mathematics, 06.01.2021 21:30


World Languages, 06.01.2021 21:30



Chemistry, 06.01.2021 21:30


Mathematics, 06.01.2021 21:30




Mathematics, 06.01.2021 21:30


Advanced Placement (AP), 06.01.2021 21:30

English, 06.01.2021 21:30

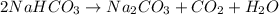
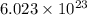 of particles.
of particles.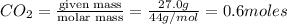
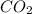 is produced from 2 moles of
is produced from 2 moles of 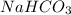
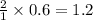 moles of
moles of