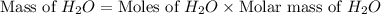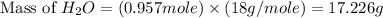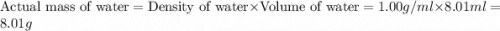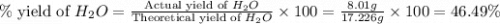Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 16:50
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1
You know the right answer?
What is the percent yield of a reaction in which 74.1 g of tungsten(vi) oxide (wo3) reacts with exce...
Questions



French, 07.03.2020 06:18


Mathematics, 07.03.2020 06:19


Mathematics, 07.03.2020 06:20


Advanced Placement (AP), 07.03.2020 06:20











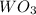 = 74.1 g
= 74.1 g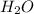 = 18 g/mole
= 18 g/mole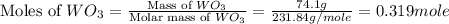
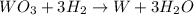
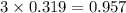 moles of
moles of