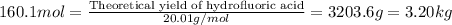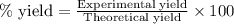Chemistry, 09.07.2019 00:50 robertschulte116
Hydrogen fluoride is used in the manufacture of freons (which destroy ozone in the stratosphere) and in the production of aluminum metal. it is prepared by the reaction caf2 + h2so4 → caso4 + 2hf in one process, 6.25 kg of caf2 is treated with an excess of h2so4 and yields 2.35 kg of hf. calculate the percent yield of hf. % yield

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1
You know the right answer?
Hydrogen fluoride is used in the manufacture of freons (which destroy ozone in the stratosphere) and...
Questions


Chemistry, 10.06.2021 18:40



Mathematics, 10.06.2021 18:40

Mathematics, 10.06.2021 18:40



Mathematics, 10.06.2021 18:40

Geography, 10.06.2021 18:40


Mathematics, 10.06.2021 18:40


English, 10.06.2021 18:40

Mathematics, 10.06.2021 18:40


Mathematics, 10.06.2021 18:40

Mathematics, 10.06.2021 18:40


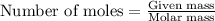 ....(1)
....(1) 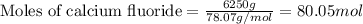
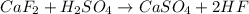
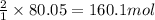 of hydrofluoric acid
of hydrofluoric acid