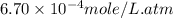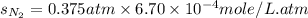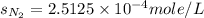Chemistry, 09.07.2019 01:30 glydelxc2780
Calculate the solubility of nitrogen in water at an atmospheric pressure of 0.480 atm (a typical value at high altitude). atmospheric gas mole fraction kh mol/(l*atm) n2 7.81 x 10-1 6.70 x 10-4 o2 2.10 x 10-1 1.30 x 10-3 ar 9.34 x 10-3 1.40 x 10-3 co2 3.33 x 10-4 3.50 x 10-2 ch4 2.00 x 10-6 1.40 x 10-3 h2 5.00 x 10-7 7.80 x 10-4

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1


Chemistry, 23.06.2019 01:00
Which elements are found in glucose, the product of photosynthesis? a. carbon, hydrogen, and oxygen b. carbon and hydrogen c. carbon, nitrogen, and oxygen d. hydrogen, nitrogen, and carbon
Answers: 2
You know the right answer?
Calculate the solubility of nitrogen in water at an atmospheric pressure of 0.480 atm (a typical val...
Questions









Geography, 07.01.2020 23:31

Computers and Technology, 07.01.2020 23:31




Social Studies, 07.01.2020 23:31



History, 07.01.2020 23:31

Social Studies, 07.01.2020 23:31


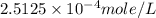
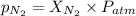
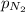 = partial pressure of nitrogen = ?
= partial pressure of nitrogen = ?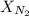 = mole fraction of nitrogen =
= mole fraction of nitrogen = 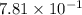
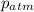 = atmospheric pressure = 0.480 atm
= atmospheric pressure = 0.480 atm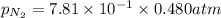
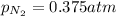
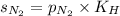
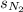 = solubility of nitrogen in water = ?
= solubility of nitrogen in water = ?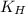 = Henry's constant =
= Henry's constant =