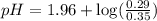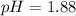Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the energy released per kilogram of fuel used. given mev per reaction, calculate energy in joules per kilogram of reactants. consider 1 mole of tritium plus 1 mole of deuterium to be a mole of “reactions” (total molar mass = 5 grams).
Answers: 1


Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3
You know the right answer?
Question 5 a solution is prepared at 25°c that is initially 0.35m in chlorous acid hclo2, a weak aci...
Questions

English, 05.12.2020 03:10

Mathematics, 05.12.2020 03:10

Physics, 05.12.2020 03:10


Mathematics, 05.12.2020 03:10

World Languages, 05.12.2020 03:10

Chemistry, 05.12.2020 03:10

Mathematics, 05.12.2020 03:10

Mathematics, 05.12.2020 03:10

Business, 05.12.2020 03:10


Chemistry, 05.12.2020 03:10


Advanced Placement (AP), 05.12.2020 03:10

Mathematics, 05.12.2020 03:10

Biology, 05.12.2020 03:10

Mathematics, 05.12.2020 03:10


Mathematics, 05.12.2020 03:10

Mathematics, 05.12.2020 03:10


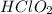 = 0.35 M
= 0.35 M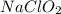 = 0.29 M
= 0.29 M .
.![pK_a=-\log [K_a]](/tpl/images/0067/9400/2d95a.png)
 in this expression, we get:
in this expression, we get: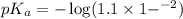
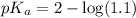
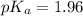
![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0067/9400/e961a.png)
![pH=pK_a+\log \frac{[NaClO_2]}{[HClO_2]}](/tpl/images/0067/9400/a8df0.png)