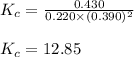Chemistry, 09.07.2019 04:30 dakotalynnwillis01
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.350 m , [b] = 0.650 m , and [c] = 0.300 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.220 m and [c] = 0.430 m . calculate the value of the equilibrium constant, kc.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

You know the right answer?
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.350 m , [b] = 0.65...
Questions


Mathematics, 21.01.2021 18:30

Mathematics, 21.01.2021 18:30

Mathematics, 21.01.2021 18:30









Arts, 21.01.2021 18:30

Mathematics, 21.01.2021 18:30

English, 21.01.2021 18:30

History, 21.01.2021 18:30


History, 21.01.2021 18:30


English, 21.01.2021 18:30

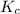 for the given reaction is 12.85.
for the given reaction is 12.85.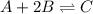
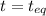 (0.350 - x) (0.650 - 2x) (0.300 + x)
(0.350 - x) (0.650 - 2x) (0.300 + x)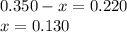
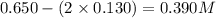
![K_c=\frac{[C]}{[A][B]^2}](/tpl/images/0068/1022/240ef.png)