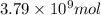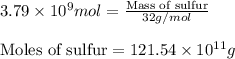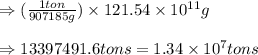Be sure to answer all parts. the annual production of sulfur dioxide from burning coal and fossil fuels, auto exhaust, and other sources is about 26 million tons. the equation for the reaction is s(s) + o2(g) → so2(g) if 2.68 × 107 tons of sulfur dioxide formed, how many tons of sulfur were present in the original materials? assume 100% yield. × 10 tons enter your answer in scientific notation.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:30
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3

Chemistry, 23.06.2019 06:30
Which of the following is true about the products formed during photosynthesis? (5 points) select one: a. they have the same mass as the mass of reactants. b. they are the same set of compounds as the reactants. c. they have more mass than the mass of reactants. d. they are chemically the same as the reactants.
Answers: 1

Chemistry, 23.06.2019 06:30
Acertain atom has 22 protons and 19 electrons. this atom loses an electron. the net charge on the atom is now 4+1+01-4-. if this same atom with 22 protons and 19 electrons were to gain 3 electrons, the net charge on the atom would be 3+2+02-3-.
Answers: 1

Chemistry, 23.06.2019 09:30
How many moles of na2s2o3 are needed to react with 0.12mol of cl2? show work.
Answers: 1
You know the right answer?
Be sure to answer all parts. the annual production of sulfur dioxide from burning coal and fossil fu...
Questions

Spanish, 03.07.2019 04:00


History, 03.07.2019 04:00

Mathematics, 03.07.2019 04:00


Mathematics, 03.07.2019 04:00




History, 03.07.2019 04:00

Mathematics, 03.07.2019 04:00

Computers and Technology, 03.07.2019 04:00

Physics, 03.07.2019 04:00

Social Studies, 03.07.2019 04:00






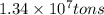
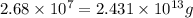
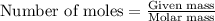 ......(2)
......(2)
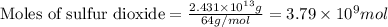
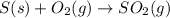
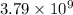 moles of sulfur dioxide will be produced from =
moles of sulfur dioxide will be produced from = 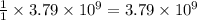 moles of sulfur.
moles of sulfur.