
Ammonia is a principal nitrogen fertilizer. it is prepared by the reaction between hydrogen and nitrogen. 3h2(g) + n2(g) → 2nh3(g) in a particular reaction, 8.00 moles of nh3 were produced. how many moles of h2 and how many moles of n2 were reacted to produce this amount of nh3?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 18:00
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1


You know the right answer?
Ammonia is a principal nitrogen fertilizer. it is prepared by the reaction between hydrogen and nitr...
Questions


Biology, 18.02.2021 14:00


Mathematics, 18.02.2021 14:00





Physics, 18.02.2021 14:00



SAT, 18.02.2021 14:00


History, 18.02.2021 14:00

Geography, 18.02.2021 14:00


Biology, 18.02.2021 14:00



Mathematics, 18.02.2021 14:00

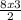 = 12moles
= 12moles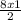 = 4moles of Nitrogen gas
= 4moles of Nitrogen gas

