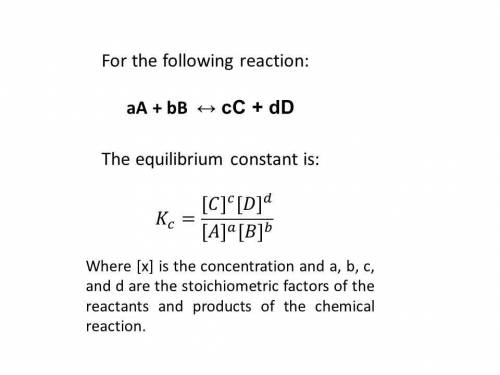
Consider the following reaction and its equilibrium constant: 4 cuo(s) + ch4(g) ⇌ co2(g) + 4 cu(s) + 2 h2o(g) kc = 1.10.
a reaction mixture contains 0.22 m ch4, 0.70 m co2 and 1.5 m h2o. which of the following statements is true concerning this system? a. the reaction will shift in the direction of products. b. the equilibrium constant will increase. c. the reaction will shift in the direction of reactants. d. the system is at equilibrium.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1
You know the right answer?
Consider the following reaction and its equilibrium constant: 4 cuo(s) + ch4(g) ⇌ co2(g) + 4 cu(s)...
Questions

Advanced Placement (AP), 24.07.2019 00:00





Mathematics, 24.07.2019 00:00


History, 24.07.2019 00:00

Chemistry, 24.07.2019 00:00









Health, 24.07.2019 00:00


![Q = \frac{[CO_{2}][H_{2}O]^{2}}{[CH_{4}]}](/tpl/images/0072/0904/ac4b6.png) (2)
(2)![Q = \frac{[CO_{2}][H_{2}O]^{2}}{[CH_{4}]} = \frac{0.70*(1.5)^{2}}{0.22} = 7.16](/tpl/images/0072/0904/5a31b.png)