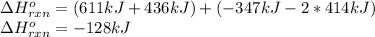Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1
You know the right answer?
Use the bond energies provided to estimate δh°rxn for the reaction below. c2h4(g) + h2(g) → c2h6(g)...
Questions

Physics, 13.01.2021 23:50






English, 13.01.2021 23:50


Mathematics, 13.01.2021 23:50

Mathematics, 13.01.2021 23:50

Mathematics, 13.01.2021 23:50

Mathematics, 13.01.2021 23:50

Mathematics, 13.01.2021 23:50

Biology, 13.01.2021 23:50

Mathematics, 13.01.2021 23:50


Health, 13.01.2021 23:50

History, 13.01.2021 23:50

Mathematics, 13.01.2021 23:50

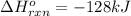
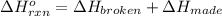
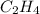 a double bond between carbons is broken as well as a bond between hydrogens (such values turn out positive). Furthermore, a single bond between carbons and two single bonds between carbon and hydrogen are made (such values turn out negative), in such a way, we develop the aforesaid equation to obtain:
a double bond between carbons is broken as well as a bond between hydrogens (such values turn out positive). Furthermore, a single bond between carbons and two single bonds between carbon and hydrogen are made (such values turn out negative), in such a way, we develop the aforesaid equation to obtain: