Chemistry, 11.07.2019 22:10 Franklyn3220
An sample of water with a mass of 123.00 kg is heated from 25 c to 97 c. if the specific heat of water is 1 j-1 kg k-1 then how much energy is required?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Describe the chemical reaction based on the chemical equation below. also, explain whether the equation is balanced.
Answers: 1

Chemistry, 22.06.2019 00:00
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3

Chemistry, 22.06.2019 00:30
Sarah wants to know where in her garden chamomile would grow the best. she thinks chamomile will grow best in the corner of the garden that gets the most sunlight. to test her hypothesis, she decides to plant several groups of chamomile in her garden as an experiment. which of the following variables will sarah need to measure to know which group of plants grew best? a. the location of the plants b. the type of plants c. the height of the plants d. the amount of water she gives the plants
Answers: 1

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3
You know the right answer?
An sample of water with a mass of 123.00 kg is heated from 25 c to 97 c. if the specific heat of wat...
Questions

Mathematics, 26.08.2019 03:30

Geography, 26.08.2019 03:30

Mathematics, 26.08.2019 03:30

Physics, 26.08.2019 03:30

Business, 26.08.2019 03:30

Health, 26.08.2019 03:30

Mathematics, 26.08.2019 03:30

Mathematics, 26.08.2019 03:30



Social Studies, 26.08.2019 03:30

English, 26.08.2019 03:30

History, 26.08.2019 03:30


Mathematics, 26.08.2019 03:50


History, 26.08.2019 03:50


English, 26.08.2019 03:50

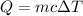
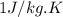
 = change in temperature =
= change in temperature = 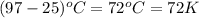 (change remains the same)
(change remains the same)