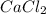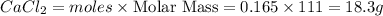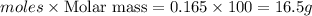When calcium carbonate is added to hydrochloric acid, calcium chloride, carbon dioxide, and water are produced.
caco3 + 2hcl ⟶cacl2 + h2o + co2
a) how many grams of calcium chloride will be produced when 26.0g of calcium carbonate are combined whith 12.0g of hydrochloric acid?
b) which reactant is in excess and how many grams of this reactant will remain after the reaction is complete?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:40
Darla claims that the first periodic table developed by mendeleev was not completely accurate, so it is not useful at all. harmony argues that it establish the periodic table we use today, making it more credible. who is correct and why? darla is correct, because a model that has any mistakes should be thrown out. darla is correct, because a good model would not need to change. harmony is correct, because mendeleev’s model had all of the information correct in the first version. harmony is correct, because mendeleev’s model made predictions that came true.
Answers: 1

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1
You know the right answer?
When calcium carbonate is added to hydrochloric acid, calcium chloride, carbon dioxide, and water ar...
Questions

Mathematics, 23.03.2021 20:30


Geography, 23.03.2021 20:30

Chemistry, 23.03.2021 20:30


Mathematics, 23.03.2021 20:30

Biology, 23.03.2021 20:30

Mathematics, 23.03.2021 20:30




Mathematics, 23.03.2021 20:30

English, 23.03.2021 20:30

Engineering, 23.03.2021 20:30






Mathematics, 23.03.2021 20:30

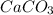 is the excess reagent and 16.5g of
is the excess reagent and 16.5g of 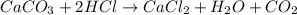
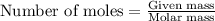
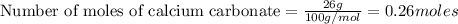
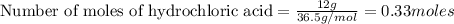
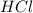 react with 1 mole of
react with 1 mole of 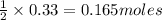 of
of