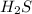Chemistry, 12.07.2019 01:30 DeGeneral770
Which of the following compounds is not likely to have ionic bonds lif, naci, ch4, mgf2

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2



Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1
You know the right answer?
Which of the following compounds is not likely to have ionic bonds lif, naci, ch4, mgf2...
Questions

English, 18.03.2021 19:20


Mathematics, 18.03.2021 19:20

Mathematics, 18.03.2021 19:20

Advanced Placement (AP), 18.03.2021 19:20


History, 18.03.2021 19:20

English, 18.03.2021 19:20

English, 18.03.2021 19:20


Mathematics, 18.03.2021 19:20

Mathematics, 18.03.2021 19:20




Mathematics, 18.03.2021 19:20



Spanish, 18.03.2021 19:20

English, 18.03.2021 19:20

 does not have ionic bonds. Because of the close value of electronegativity of the carbon and hydrogen atoms the electrons are shared forming covalent bonds.
does not have ionic bonds. Because of the close value of electronegativity of the carbon and hydrogen atoms the electrons are shared forming covalent bonds.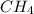 is not likely to have ionic bonds
is not likely to have ionic bonds 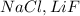 and
and 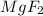 are examples in which we see metal bonded with a non-metal.
are examples in which we see metal bonded with a non-metal.