
Chemistry, 12.07.2019 02:20 Cupcake8189
Part 1. determine the molar mass of a 0.622-gram sample of gas having a volume of 2.4 l at 287 k and 0.850 atm. show your work.
part 2. if this sample was placed under extremely low temperature, describe how the actual volume would compare to the predicted volume. explain your answer.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 23.06.2019 07:30
Assume that 13.5 g solid aluminum (al) react with hcl to produce solid aluminum chloride (alcl3) salt and gaseous hydrogen (h2) at standard temperature and pressure.
Answers: 1
You know the right answer?
Part 1. determine the molar mass of a 0.622-gram sample of gas having a volume of 2.4 l at 287 k and...
Questions


SAT, 15.07.2019 02:30

Mathematics, 15.07.2019 02:30

Mathematics, 15.07.2019 02:30


Mathematics, 15.07.2019 02:30



Mathematics, 15.07.2019 02:30

Mathematics, 15.07.2019 02:30



Mathematics, 15.07.2019 02:30


Social Studies, 15.07.2019 02:30

Mathematics, 15.07.2019 02:30


Mathematics, 15.07.2019 02:30

Biology, 15.07.2019 02:30

Biology, 15.07.2019 02:30

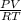
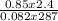
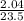 = 0.087mole
= 0.087mole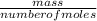
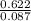 =7.15gmol⁻¹
=7.15gmol⁻¹

