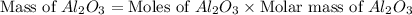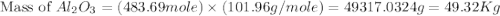Chemistry, 12.07.2019 03:20 deaerionharper
2al(s)+fe2o3(s)−→−heatal2o3(s)+2fe( l) 2al(s)+fe2o3(s)→heatal2o3(s)+2fe(l) if 26.1 kg al26.1 kg al reacts with an excess of fe2o3,fe2o3, how many kilograms of al2o3al2o3 will be produced?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 23.06.2019 01:00
What type of chemical bond is formed between two atoms of bromine 1. metallic 2. hydrogen 3. ionic 4. covalent
Answers: 1

Chemistry, 23.06.2019 02:30
Which of the four hypothetical substances you investigated would be most harmful to living organisms? 50 points!
Answers: 2
You know the right answer?
2al(s)+fe2o3(s)−→−heatal2o3(s)+2fe( l) 2al(s)+fe2o3(s)→heatal2o3(s)+2fe(l) if 26.1 kg al26.1 kg al r...
Questions


Mathematics, 29.01.2020 21:59

Health, 29.01.2020 21:59

Mathematics, 29.01.2020 21:59

Mathematics, 29.01.2020 21:59

History, 29.01.2020 21:59

Mathematics, 29.01.2020 21:59








Mathematics, 29.01.2020 21:59

English, 29.01.2020 21:59




Mathematics, 29.01.2020 21:59

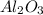 produced will be, 49.32 Kg
produced will be, 49.32 Kg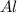 = 26.1 Kg = 26100 g
= 26.1 Kg = 26100 g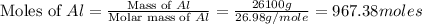
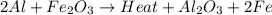
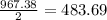 moles of
moles of