

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1

You know the right answer?
The combustion of propane (c3h8) produces co2 and h2o: c3h8 (g) 5o2 (g) → 3co2 (g) 4h2o (g) the rea...
Questions

Mathematics, 20.12.2019 23:31




Mathematics, 20.12.2019 23:31

Mathematics, 20.12.2019 23:31


Spanish, 20.12.2019 23:31




English, 20.12.2019 23:31



Mathematics, 20.12.2019 23:31


Mathematics, 20.12.2019 23:31



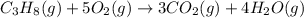
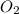 produces = 4 moles of
produces = 4 moles of 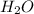
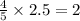 moles of
moles of 

