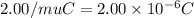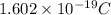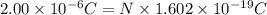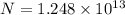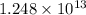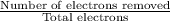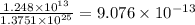Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2


You know the right answer?
A50.0-g ball of copper has a net charge of 2.00 µc . what fraction of the copper’s electrons has bee...
Questions



Biology, 24.07.2019 09:00

Mathematics, 24.07.2019 09:00


English, 24.07.2019 09:00

Social Studies, 24.07.2019 09:00


Spanish, 24.07.2019 09:00

Mathematics, 24.07.2019 09:00

Chemistry, 24.07.2019 09:00


History, 24.07.2019 09:00







 .
.
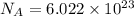
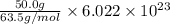
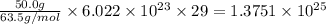
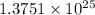 ....(1)
....(1)