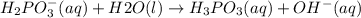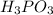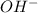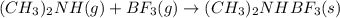Using the br? nsted-lowry concept of acids and bases, identify the br? nsted-lowry acid and base in each of the following reactions:
h2po3? (aq)+h2o(l)? h3po3(aq)+oh? (aq)
(ch3)2nh(g)+bf3()2nhbf3(s)
drag the appropriate items to their respective bins.
h2po3- h2o bf3 (ch3)2nh
bronsted lowry acid bronsted lowry base neither

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1


Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1

You know the right answer?
Using the br? nsted-lowry concept of acids and bases, identify the br? nsted-lowry acid and base in...
Questions


Social Studies, 02.02.2020 17:45


Mathematics, 02.02.2020 17:45


Mathematics, 02.02.2020 17:45

Mathematics, 02.02.2020 17:45

Physics, 02.02.2020 17:45



Mathematics, 02.02.2020 17:45

Mathematics, 02.02.2020 17:45

Mathematics, 02.02.2020 17:45

Mathematics, 02.02.2020 17:45

Mathematics, 02.02.2020 17:45



Mathematics, 02.02.2020 17:45

Biology, 02.02.2020 17:45

Mathematics, 02.02.2020 17:45

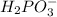 is Bronsted Lowry base.
is Bronsted Lowry base.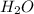 is Bronsted Lowry acid.
is Bronsted Lowry acid.