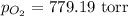Asample of oxygen gas was collected via water displacement. since the oxygen was collected via water displacement, the sample is saturated with water vapor. if the total pressure of the mixture at 26.4 °c is 805 torr, what is the partial pressure of oxygen? the vapor pressure of water at 26.4 °c is 25.81 mm hg.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 07:00
6what is the importance of water on earth? a) it keeps the top layer of the geosphere cool b) it allows life to exist c) it provides ice at the poles d) it creates earth's blue color from space
Answers: 2

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1
You know the right answer?
Asample of oxygen gas was collected via water displacement. since the oxygen was collected via water...
Questions


Spanish, 17.07.2019 08:00

Health, 17.07.2019 08:00


Mathematics, 17.07.2019 08:00

Mathematics, 17.07.2019 08:00








Health, 17.07.2019 08:00

History, 17.07.2019 08:00


History, 17.07.2019 08:00



Social Studies, 17.07.2019 08:00

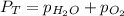
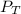 = total partial pressure = 805 torr
= total partial pressure = 805 torr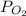 = partial pressure of oxygen gas = ?
= partial pressure of oxygen gas = ?
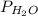 = partial pressure of water = 25.81 mm Hg = 25.81 torr
= partial pressure of water = 25.81 mm Hg = 25.81 torr