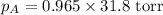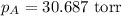Chemistry, 12.07.2019 18:10 damaricoleman42
Calculate the vapor pressure of a solution containing 24.6 g of glycerin (c3h8o3) in 134 ml of water at 30.0 ∘c. the vapor pressure of pure water at this temperature is 31.8 torr. assume that glycerin is not volatile and dissolves molecularly (i. e., it is not ionic) and use a density of 1.00 g/ml for the water.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 23.06.2019 00:00
How is the way a mixture is combined different from how a compound is combined?
Answers: 3


Chemistry, 23.06.2019 13:30
What happens to acetone molecules when you add heat to a beaker of liquid acetone?
Answers: 1
You know the right answer?
Calculate the vapor pressure of a solution containing 24.6 g of glycerin (c3h8o3) in 134 ml of water...
Questions

Computers and Technology, 23.06.2019 21:20













Mathematics, 23.06.2019 21:20

Mathematics, 23.06.2019 21:20


Mathematics, 23.06.2019 21:20



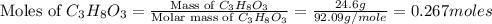
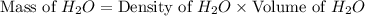
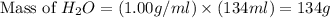
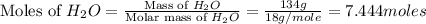
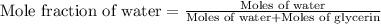
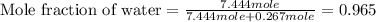
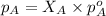
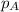 = vapor pressure of solution = ?
= vapor pressure of solution = ?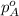 = vapor pressure of pure water= 31.8 torr
= vapor pressure of pure water= 31.8 torr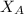 = mole fraction of water = 0.965
= mole fraction of water = 0.965