
The bronsted-lowry definition of acids and bases refers to the transfer of a "proton" from the acid to the base; however, the symbol for a proton (p+) is not generally used in this context. what is the chemical symbol that is commonly used to represent a "proton" in the context of bronsted-lowry acids and bases?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Imagine that you’re getting ready to move to a new city. when people move, they are influenced by push factors and pull factors, and you have many reasons for your move. which of the following factors is an example of a pull factor? a. wanting to move because you’ve found a great new school somewhere new b. needing to move because there are not enough resources in your old hometown c. being forced to move because your old home is gone d. having to move because there are no jobs in your current hometown
Answers: 1

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2
You know the right answer?
The bronsted-lowry definition of acids and bases refers to the transfer of a "proton" from the acid...
Questions


Mathematics, 13.02.2021 02:00

Mathematics, 13.02.2021 02:00


Mathematics, 13.02.2021 02:00


Mathematics, 13.02.2021 02:00



Mathematics, 13.02.2021 02:00

Chemistry, 13.02.2021 02:00


Mathematics, 13.02.2021 02:00

Mathematics, 13.02.2021 02:00

English, 13.02.2021 02:00


Physics, 13.02.2021 02:00

Mathematics, 13.02.2021 02:00

Mathematics, 13.02.2021 02:00

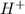
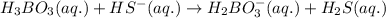
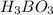 is loosing a proton, thus it is considered as an acid and after losing a proton, it forms
is loosing a proton, thus it is considered as an acid and after losing a proton, it forms 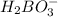 which is a conjugate base.
which is a conjugate base.
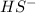 is gaining a proton, thus it is considered as a base and after gaining a proton, it forms
is gaining a proton, thus it is considered as a base and after gaining a proton, it forms 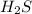 which is a conjugate acid.
which is a conjugate acid.


