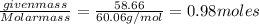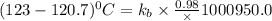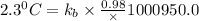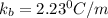Chemistry, 12.07.2019 20:30 joannegrace869
The normal boiling point of a certain liquid x is 120.7°c , but when 58.66g of urea nh22co are dissolved in 950.g of x , it is found that the solution boils at 123.0°c instead. use this information to calculate the molal boiling point elevation constant kb of x .

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 22.06.2019 17:30
Aroller coaster is traveling at 13 mi./s when you purchase a hill that is 400 m long and down the hill exonerate at 4.0 m/s squared what is the final velocity of the posterior found your answer to the nearest number
Answers: 1
You know the right answer?
The normal boiling point of a certain liquid x is 120.7°c , but when 58.66g of urea nh22co are disso...
Questions




















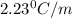
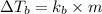
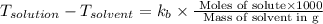
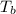 = change in boiling point
= change in boiling point
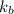 = boiling point constant
= boiling point constant