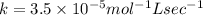Chemistry, 12.07.2019 21:30 mxddisonxo
The reaction between ethyl bromide (c2h5br) and hydroxide ion in ethyl alcohol at 330 k, c2h5br(alc) + oh-(alc) --> c2h5oh(l) + br-(alc), is first order each in ethyl bromide and hydroxide ion. when [c2h5br] is 0.0477 m and [oh-] is 0.100 m, the rate of disappearance of ethyl bromide is 1.7 x 10^-7 m/s.
what is the value of the rate constant?
k=?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1


Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2
You know the right answer?
The reaction between ethyl bromide (c2h5br) and hydroxide ion in ethyl alcohol at 330 k, c2h5br(alc)...
Questions

English, 06.07.2019 05:30







Mathematics, 06.07.2019 05:30

Arts, 06.07.2019 05:30

Mathematics, 06.07.2019 05:30



Spanish, 06.07.2019 05:30


Mathematics, 06.07.2019 05:30

History, 06.07.2019 05:30

Biology, 06.07.2019 05:30



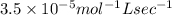
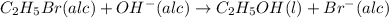
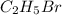 = 1
= 1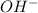 = 1
= 1![Rate=k[C_2H_5Br]^1[OH^-]^1](/tpl/images/0082/3546/7f75e.png)
![Rate=-\frac{1d[C_2H_5Br]}{dt}=k[C_2H_5Br]^1[OH^-]^1](/tpl/images/0082/3546/b56e9.png)
![\frac{d[C_2H_5]}{dt}]=1.7\times 10^{-7}](/tpl/images/0082/3546/5e05d.png)
![Rate=1.7\times 10^{-7}=k[0.0477]^1[0.100]^1](/tpl/images/0082/3546/1123d.png)