Chemistry, 12.07.2019 23:30 dbn4everloved
Liquid octane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . suppose 97. g of octane is mixed with 150. g of oxygen. calculate the maximum mass of water that could be produced by the chemical reaction. round your answer to significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 20:00
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3
You know the right answer?
Liquid octane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . s...
Questions


Mathematics, 29.11.2020 14:10


Biology, 29.11.2020 14:10



Mathematics, 29.11.2020 14:10

Biology, 29.11.2020 14:10

Computers and Technology, 29.11.2020 14:10











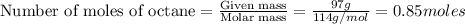
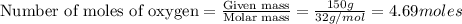
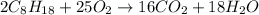
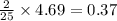 moles of octane
moles of octane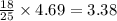 moles of water.
moles of water.