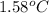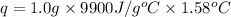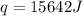Chemistry, 13.07.2019 01:30 jmanrules200
When 1.0 g of fructose, c6h12o6(s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the calorimeter increases by 1.58 °c. if the heat capacity of the calorimeter and its contents is 9.90 kj/°c, what is q for this combustion?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration
Answers: 1

Chemistry, 22.06.2019 01:30
Aroller coaster car is traveling down a track at 22 m/s. the car has a mass of 2000 kg. what is the kinetic energy of the car? a) 22,000 j b) 968,000 j c) 484,000 j d) 44,000 j
Answers: 2

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1
You know the right answer?
When 1.0 g of fructose, c6h12o6(s), a sugar commonly found in fruits, is burned in oxygen in a bomb...
Questions



English, 13.04.2021 17:40


Mathematics, 13.04.2021 17:40

Mathematics, 13.04.2021 17:40

Spanish, 13.04.2021 17:40


Social Studies, 13.04.2021 17:40

Geography, 13.04.2021 17:40

Biology, 13.04.2021 17:40

History, 13.04.2021 17:40



Engineering, 13.04.2021 17:40





Social Studies, 13.04.2021 17:40

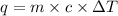
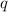 = heat of combustion = ?
= heat of combustion = ?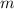 = mass of fructose = 1.0 g
= mass of fructose = 1.0 g = heat capacity of the calorimteter =
= heat capacity of the calorimteter = 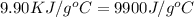
 = change in temperature =
= change in temperature =