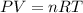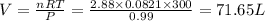Automobile air bags are inflated with nitrogen gas, which is formed by the decomposition of solid sodium azide (nan3). the other product is sodium metal. calculate the volume of nitrogen gas at 27 °c and 756 torr formed by the decomposition of 125 g of sodium azide.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 03:30
What is the number of moles of chemical units represented by 9.03x10^24? and how do i show work? (dumb it down )
Answers: 1

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2
You know the right answer?
Automobile air bags are inflated with nitrogen gas, which is formed by the decomposition of solid so...
Questions

Biology, 20.10.2019 10:10

History, 20.10.2019 10:10



Mathematics, 20.10.2019 10:10

Mathematics, 20.10.2019 10:10


History, 20.10.2019 10:10

English, 20.10.2019 10:10




Arts, 20.10.2019 10:10

Mathematics, 20.10.2019 10:10






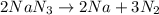
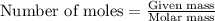
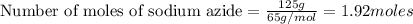
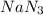 produce 3 moles of
produce 3 moles of 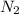
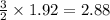 moles of
moles of